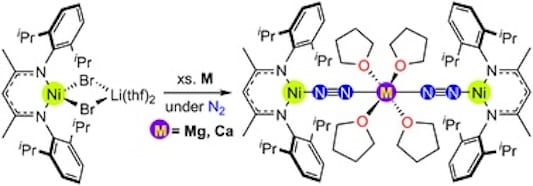
89. Alkaline Earth Metal-Assisted Dinitrogen Activation at Nickel
Knoell, T.; Polanco, J.; MacMillan, S.N.; Bertke, J.A.; Foroutan-Nejad, C.; Lancaster, K.M.; Bakhoda, A. Dalton Trans. 2023, 53, 4689-4697.
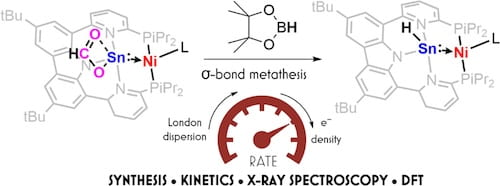
88. Accelerating σ-Bond Metathesis at Sn(II) Centers
Kong, R.Y.; Parry, J.B.; Anello, G.R.; Ong, M.E., Lancaster, K.M. J. Am. Chem. Soc. 2023, 145, 24136–24144.
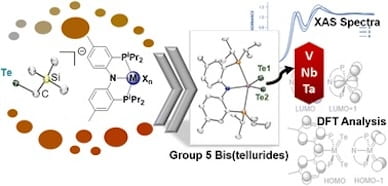
87. Tellurolate: An Effective Te-atom Transfer Reagent to Prepare the Triad of Group 5 Metal Bis(tellurides)
Senthil, S.; Kwon, S.; Kong, R.Y.; MacMillan, S.N.; Zatsepin, P.; Gau, M.R.; Carroll, P.J.; Baik, M-H.; Lancaster, K.M.; Mindiola, D.J. Chem. Sci. 2023, 14, 12277-12282 .
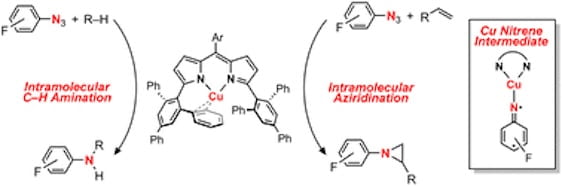
86. Nitrene Transfer From a Sterically Confined Copper Nitrenoid Dipyrrin Complex
Carsch, K.M.; North, S.C.; DiMucci, I.M.; Iliescu, A.; Vojáčková, P.; Khazanov, T.; Zheng, S-L.; Cundari, T.R.; Lancaster, K.M.; Betley, T.A. Chem. Sci. 2023, 14, 10847-10860.
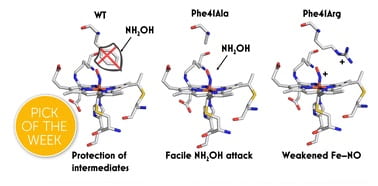
85. Outer Coordination Sphere Influences on Cofactor Maturation and Substrate Oxidation by Cytochrome P460
Bollmeyer, M.M.; Majer, S.H.; Coleman, R.E.; Lancaster, K.M. Chem. Commun. 2023, 14, 8295–8304.
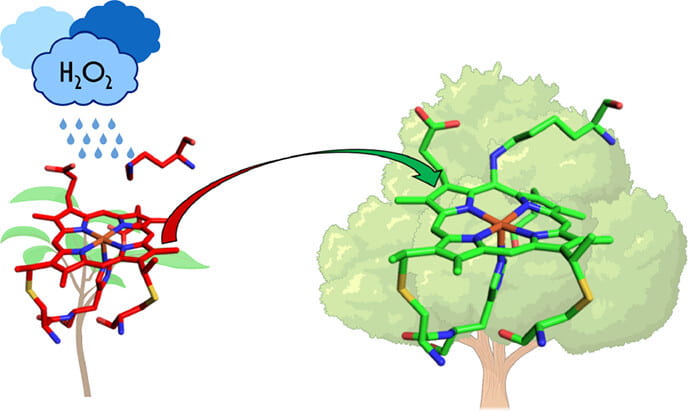
84. Cytochrome P460 Cofactor Maturation Proceeds via Peroxide-Dependent Post-translational Modification
Bollmeyer, M.M.; Coleman, R.E.; Majer, S.H.; Ferrao, S.D.; Lancaster, K.M. J. Am. Chem. Soc. 2023, 145, 14404–14416.
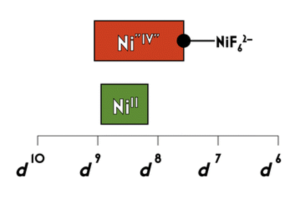
83. Scrutinizing Formally NiIV Centers Through the Lenses of Core Spectroscopy, Molecular Orbital Theory, and Valence Bond Theory
DiMucci, I.M.; Titus, C.J.; Nordlund, D.; Bour, J.R.; Chong, E.; Grigas, D.P.; Hu, C-H.; Kosobokov, M.D.; Martin, C.D.; Mirica, L.M.; Nebra, N.; Vicic, D.A.; Yorks, L.L.; Yruegas, S.; MacMillan, S.N.; Shearer, J.; Lancaster, K.M. Chem. Sci. 2023, 14, 6915–6929.
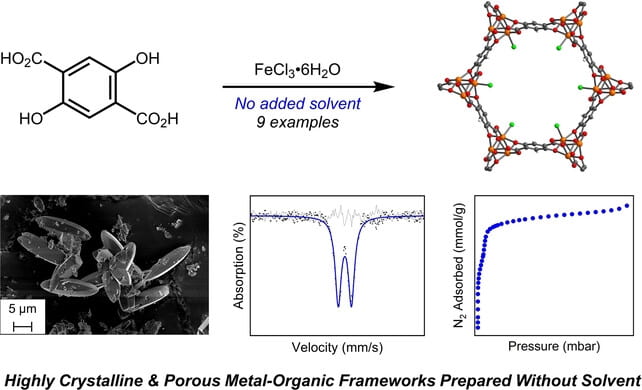
82. Ionothermal Synthesis of Metal-Organic Frameworks Using Low-Melting Metal Salt Precursors
Azbell, T.J.; Pitt, T.A.; Bollmeyer, M.M.; Cong, C.; Lancaster, K.M.; Milner, P.J. Angew. Chem. Int. Ed. 2023, 62, e202218252.
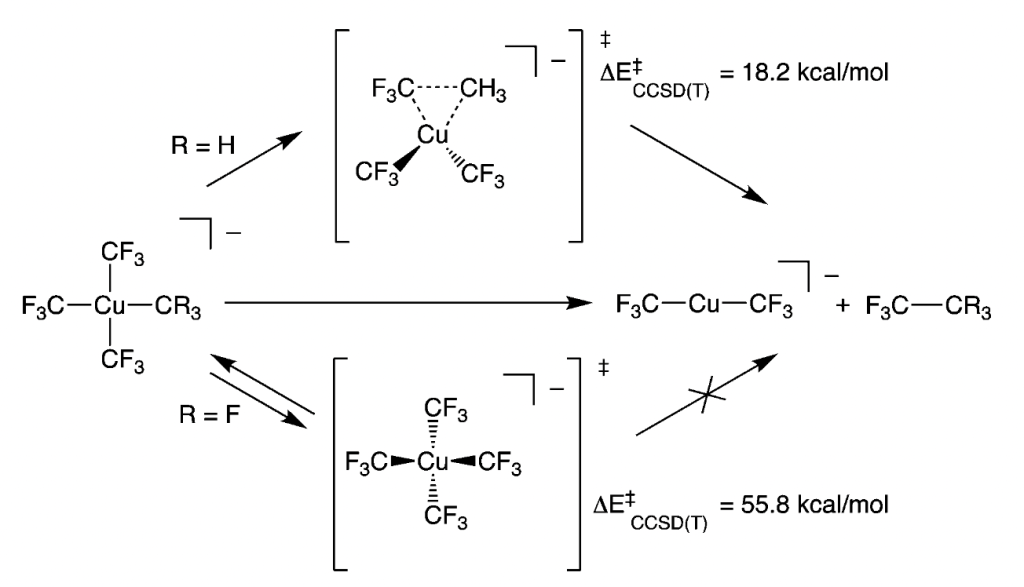
81. Bonding and the Role of Electrostatics in Driving C–C bond Formation in High Valent Organocopper Compounds
Shearer, J.; Vasiliauskasa, D.; Lancaster, K.M. Chem. Commun. 2023, 59, 98–101.
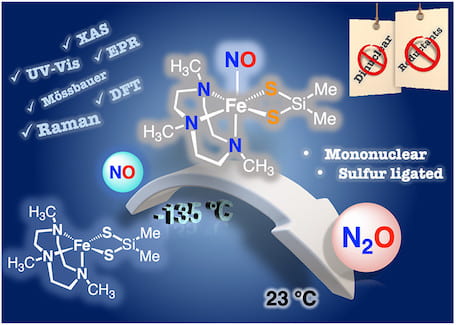
80. Direct Reduction of NO to N2O by a Mononuclear Nonheme Thiolate Ligated Iron(II) Complex via Formation of a Metastable {FeNO}7 Complex
Dey, A.; Albert, T.; Kong, R.Y.; MacMillan, S.N.; Moënne-Loccoz, P.; Lancaster, K.M.; Goldberg, D.P. Inorg. Chem. 2022, 61, 14909–14917.
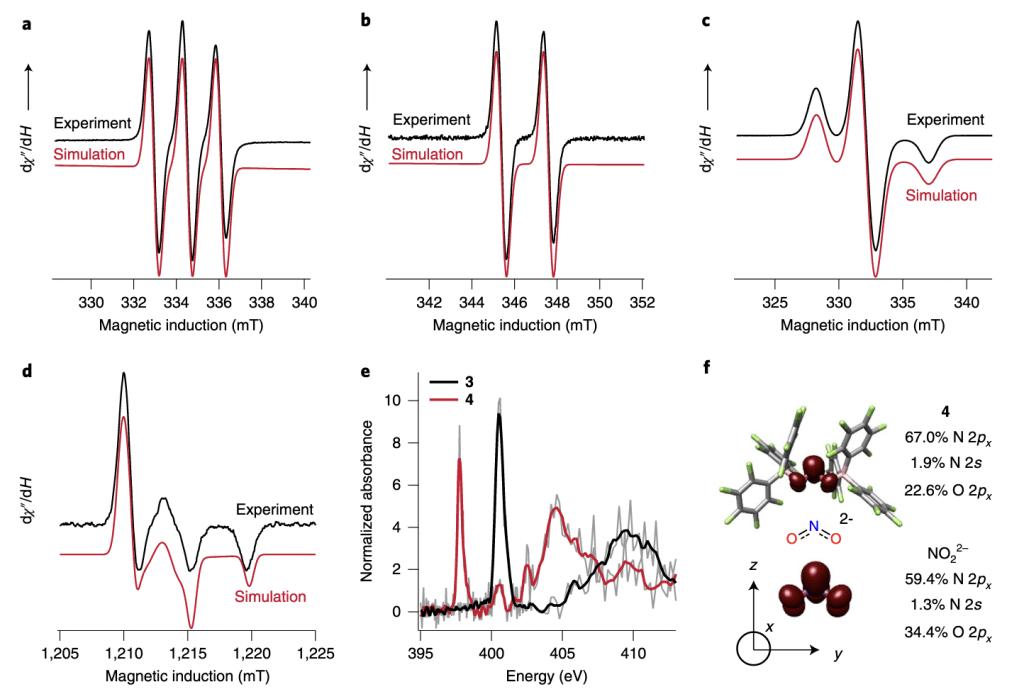
79. Lewis acid-assisted reduction of nitrite to nitric and nitrous oxides via the elusive nitrite radical dianion
Hosseininasab, V.; DiMucci, I.M.; Ghosh, P.; Bertke, J.A.; Chandrasekharan, S.; Titus, C.J.; Nordlund, D.; Freed, J.H.; Lancaster, K.M.; Warren, T.H. Nat. Chem. https://doi.org/10.1038/s41557-022-01025-9
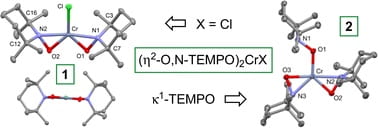
78. TEMPO coordination and reactivity in group 6; pseudo-pentagonal planar (η2-TEMPO)2CrX (X = Cl, TEMPO)
Kayser, A.K.; Wolczanski, P.T.; Cundari, T.R.; Bollmeyer, M.M.; Lancaster, K.M.; MacMillan, S.N. Chem. Commun. 2022, 58, 9818–9821.
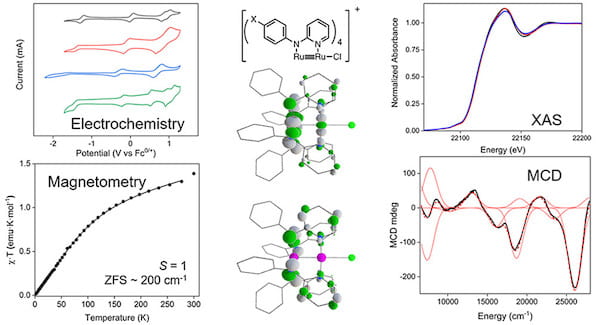
77. Electronic Structure of Ru26+ Complexes with Electron-Rich Anilinopyridinate Ligands
Roy, M.D.; Trenerry, M.J.; Thakuri, B.; MacMillan, S.N.; Liptak, M.D.; Lancaster, K.M.; Berry, J.F. Inorg. Chem. 2022, 61, 3443–3457.
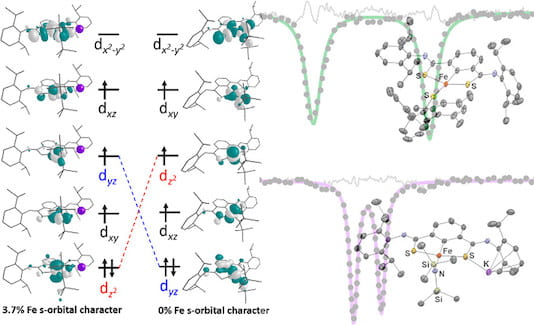
76. Iron Complexes of a Proton-Responsive SCS Pincer Ligand with a Sensitive Electronic Structure
Skubi, K.L.; Hooper, R.X.; Mercado, B.Q.; Bollmeyer, M.M.; MacMillan, S.N.; Lancaster, K.M.; Holland, P.L. Inorg. Chem. 2022, 61, 1644–1658.
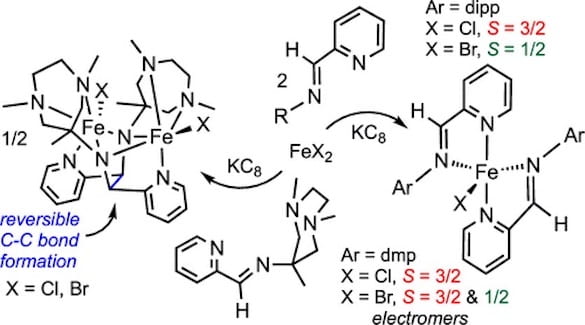
75. Reversible C–C Bond Formation, Halide Abstraction, and Electromers in Complexes of Iron Containing Redox-Noninnocent Pyridine-imine Ligands
Pokhriyal, D.; Heins, S.P.; Sifri, R.J.; Gentekos, D.T.; Coleman, R.E.; Wolczanski, P.T.; Cundari, T.R.; Fors, B.P.; Lancaster, K.M.; MacMillan, S.N. Inorg. Chem. 2021, 60, 18662-18673.
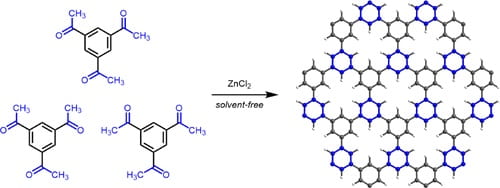
74. Conjugated Microporous Polymers via Solvent-Free Ionothermal Cyclotrimerization of Methyl Ketones
Kim, J.; Moisanu, C.M.; Gannett, C.N.; Halder, A.; Fuentes-Rivera, J.J.; Majer, S.H.; Lancaster, K.M.; Forse, A.C.; Abruña, H.D.; Milner, P.J. Chem. Mater. 2021, 33, 8334-8342.

73. Lithium superoxide encapsulated in a benzoquinone anion matrix
Nava, M.; Zhang, S.; Pastore, K.S.; Feng, X.; Lancaster, K.M.; Nocera, D.G.; Cummins, C.C. Angew. Chem. Intl. Ed. 2021, 133, e2019392118.
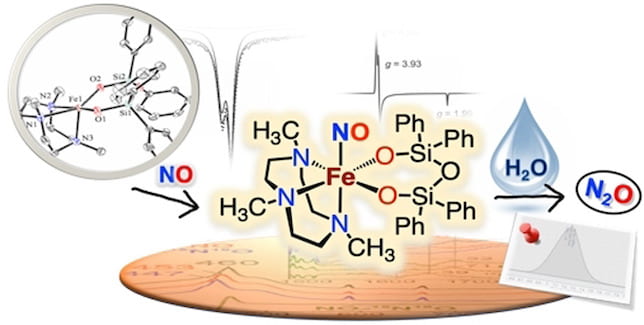
72. A Nonheme Mononuclear {FeNO}7 Complex that Produces N2O in the Absence of an Exogenous Reductant
Dey, A.; Gordon, J.B.; Albert, T.; Sabuncu, S.; Siegler, M.A.; MacMillan, S.N.; Lancaster, K.M.; Moënne-Loccoz, P.; Goldberg, D.P. Angew. Chem. Intl. Ed. 2021, 133, 21728-21724.
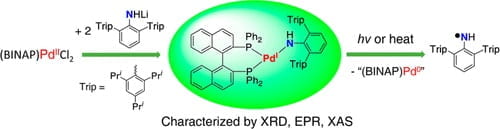
71. An Isolable Mononuclear Palladium(I) Amido Complex
Liu, J.; Bollmeyer, M.M.; Kim, Y.; Xiao, D.; MacMillan, S.N.; Chen, Q.; Leng, X.; Kim, S.H.; Zhao, L.; Lancaster, K.M.; Deng, L. J. Am. Chem. Soc. 2021, 143, 10751-10759.
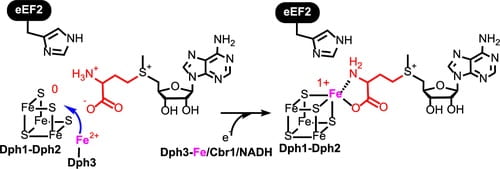
70. Dph3 Enables Aerobic Diphthamide Biosynthesis by Donating One Iron Atom to Transform a [3Fe–4S] to a [4Fe–4S] Cluster in Dph1–Dph2
Zhang, Y.; Su, D.; Dzikovski, B.; Majer, S.H.; Coleman, R.; Chandrasekaran, S.; Fenwick, M.K.; Crane, B.R.; Lancaster, K.M.; Freed, J.H.; Lin, H. J. Am. Chem. Soc. 2021, 143, 9314–9319.
Prior to 2021
69. Stein, L.Y.; Klotz, M.G.; Lancaster, K.M.; Nicol, G.W.;Qin, W.; Schleper, C.; Stahl, D.; Ward, B.B.; Yoon, S. “Comment on “A Critical Review on Nitrous Oxide Production by Ammonia-Oxidizing Archaea” by Lan Wu, Xueming Chen, Wei Wei, Yiwen Liu, Dongbo Wang, and Bing-Jie Ni.” Environ. Sci. Technol. 2020, 55, 797-798.
68. Reinholdt, A.; Pividori, D.; Laughlin, A.L.; DiMucci, I.M.; MacMillan, S.N.; Jafari, M.G.; Gau, M.R.; Carroll, P.J.; Krzystek, J.; Ozarowski, A.; Telser, J.; Lancaster, K.M.; Karsten Meyer, K.; Mindiola, D.J. “A Mononuclear and High-Spin Tetrahedral TiII Complex.” Inorg. Chem. 2020, 59, 17834-17850.
67. Coleman, R.E.; Lancaster, K.M. “Heme P460: A (Cross) Link to Nitric Oxide.” Acc. Chem. Res. 2020, 12, 2925-2935.
66. DiMucci, I.M.; MacMillan, S.N.; Walroth, R.C.; Lancaster, K.M. “Scrutinizing “Ligand Bands” via Polarized Single-Crystal X-ray Absorption Spectra of Copper(I) and Copper(II) Bis-2,2′-bipyridine Species.” Inorg. Chem. 2020, 59, 13416-13426.
65. Nagelski, A.L.; Fataftah, M.S.; Bollmeyer, M.M.; McWilliams, S.F.; MacMillan, S.N.; Mercado, B.Q.; Lancaster, K.M.; Holland, P.L. “The influences of carbon donor ligands on biomimetic multi-iron complexes for N2 reduction.” Chem. Sci. 2020, 11, 12710-12720.
64. He, X.; Looker, B. G.; Dinh, K.T.; Stubbs, A.W.; Chen, T.; Meyer, R.J.; Serna, P.; Román-Leshkov, Y.; Lancaster, K.M.; Dincă, M. “Cerium(IV) Enhances the Catalytic Oxidation Activity of Single-Site Cu Active Sites in MOFs.” ACS Catal. 2020, 10, 7820-7825.
63. Wu, T.; MacMillan, S.N.; Rajabimoghadam, K.; Siegler, M.A.; Lancaster, K.M.; Garcia-Bosch, I. “Structure, Spectroscopy, and Reactivity of a Mononuclear Copper Hydroxide Complex in Three Molecular Oxidation States.” J. Am. Chem. Soc. 2020, 28, 12265-12276.
62. Shreiber, S.T.; DiMucci, I.M.; Khrizanforov, M.N.; Titus, C.J.; Nordlund, D.; Dudkina, Y.; Cramer, R.E.; Budnikova, Y.; Lancaster, K.M.; Vicic, D.A. “[(MeCN)Ni(CF3)3]− and [Ni(CF3)4]2–: Foundations toward the Development of Trifluoromethylations at Unsupported Nickel.” Inorg. Chem. 2020, 59, 9143-9151.
61. Coleman, R.E.; Vilbert, A.C.; Lancaster, K.M. “The Heme–Lys Cross-Link in Cytochrome P460 Promotes Catalysis by Enforcing Secondary Coordination Sphere Architecture.” Biochemistry 2020, 59, 2289-2298.
60. Goodwin, C.A.P; Réant, B.L.L.; Vettese, G.F.; Kragskow, J.G.C.; Giansiracusa, M.J.; DiMucci, I.M.; Lancaster, K.M.; Mills, D.P.; Sproules, S. “Heteroleptic Samarium(III) Chalcogenide Complexes: Opportunities for Giant Exchange Coupling in Bridging σ- and π-Radical Lanthanide Dichalcogenides.” Inorg. Chem. 2020, 59, 7571-7583.
59. Ferousi, C.; Majer, S.H.; DiMucci, I.M.; Lancaster, K.M. “Biological and Bioinspired Inorganic N–N Bond-Forming Reactions.” Chem. Rev. 2020, 120, 5252-5307.
58. Rathnayaka, S.C.; Islam, S.M.; DiMucci, I.M.; MacMillan, S.N.; Lancaster, K.M.; Mankad, N.P. “Probing the Electronic and Mechanistic Roles of the μ4-sulfur Atom in a Synthetic CuZ Model System.” Chem. Sci. 2020, 11, 3441-3447.
57. Carsch, K.M.; Lukens, J.T.; DiMucci, I.M.; Iovan, D.A.; Zheng, S-L.; Lancaster, K.M.; Betley, T.A. “Electronic Structures and Reactivity Profiles of Aryl Nitrenoid-Bridged Dicopper Complexes.” J. Am. Chem. Soc. 2020, 142, 2264-2276.
56. Dong, Y.; Lukens, J.T.; Clarke, R.M.; Zheng, S-L.; Lancaster, K.M.; Betley, T.A. “Synthesis, Characterization and C–H Amination Reactivity of Nickel Iminyl Complexes.” Chem. Sci. 2020, 11, 1260-1268.
55. DiMucci, I.M.; Lukens, J.T.; Chatterjee, S.; Carsch, K.M.; Titus, C.J.; Lee, S.J.; Nordlund, D.; Betley, T.A.; MacMillan, S.N.; Lancaster, K.M. “The Myth of d8 Copper(III).” J. Am. Chem. Soc. 2019, 141, 18508-18520.
54. Gordon, J.B.; Vilbert, A.C.; DiMucci, I.M.; MacMillan, S.N.; Lancaster, K.M.; Moënne-Loccoz, P.; Goldberg, D.P. “Activation of Dioxygen by a Mononuclear Nonheme Iron Complex: Sequential Peroxo, Oxo, and Hydroxo Intermediates.” J. Am. Chem. Soc. 2019, 141, 17533-17547.
53. MacLeod, K.C.; DiMucci, I.M.; Zovinka, E.P.; McWilliams, S.F.; Mercado, B.Q.; Lancaster, K.M.; Holland, P.L. “Masked Radicals: Iron Complexes of Trityl, Benzophenone, and Phenylacetylene.” Organometallics 2019, 38, 4224-4232.
52. Carsch, K.M.; DiMucci, I.M.; Iovan, D.A.; Li, A.; Zheng, S.; Titus, C.J.; Lee, S.J.; Irwin, K.D.; Nordlund, D.; Lancaster, K.M.; Betley, T.A. “Synthesis of a copper-supported triplet nitrene complex pertinent to copper-catalyzed amination.” Science 2019, 365, 1138-1143.
51. Dunn, P.L.; Chatterjee, S.; MacMillan, S.N.; Pearce, A.J.; Lancaster, K.M.; Tonks, I.A. “The 4-Electron Cleavage of a N═N Double Bond by a Trimetallic TiNi2 Complex.” Inorg. Chem. 2019, 158, 11762-11772.
50. Dempsey, J.L.; Lancaster, K.M. “Celebrating the Year of the Periodic Table: Emerging Investigators in Inorganic Chemistry.” Inorg. Chem. 2019, 58, 10433-10435.
49. Muok, A.R.; Yijie Deng, Y.; Vadim M. Gumerov, V.M.; Jenna E. Chong, J.E.; Jennifer R. DeRosa, J.R.; Kurniyati, K.; Rachael E. Coleman, R.E.; Lancaster, K.M.; Li, C; Zhulin, I.B.; Crane, B.R. “A di-iron protein recruited as an Fe[II] and oxygen sensor for bacterial chemotaxis functions by stabilizing an iron-peroxy species.” Proc. Natl. Acad. Sci. 2019, 116, 114955–14960.
48. Confer, A.M.; Vilbert, A.C.; Dey, A.; Lancaster, K.M.; Goldberg, D.P. “A Mononuclear, Nonheme FeII–Piloty’s Acid (PhSO2NHOH) Adduct: An Intermediate in the Production of {FeNO}7/8 Complexes from Piloty’s Acid.” J. Am. Chem. Soc. 2019, 141, 7046–7055.
47. Lukens, J.T.; DiMucci, I.M.; Kurogi, T.; Mindiola, D.J.; Lancaster, K.M. “Scrutinizing metal–ligand covalency and redox non-innocence via nitrogen K-edge X-ray absorption spectroscopy.” Chem. Sci. 2019, 10, 5044–5055.
46. Moore, J.T.; Chatterjee, S.; Tarrago, M.; Clouston, L.J.; Sproules, S.; Bill, E.; Bernales, V.; Gagliardi, L.; Ye, S.; Lancaster, K.M.; Lu, C.C. “Enhanced Fe-Centered Redox Flexibility in Fe–Ti Heterobimetallic Complexes.” Inorg. Chem. 2019, 58, 6199-6214.
45. Reinholdt, A.; Majer, S.H.; Gelardi, R.M.; MacMillan, S.N.; Hill, A.F.; Wendt, O.F.; Lancaster, K.M.; Bendix, J. “An Approach to Carbide-Centered Cluster Complexes.” Inorg. Chem. 2019, 58, 4812-4819.
44. Lancaster, K.M. “Editorial overview: Emergent lessons from the elements of life.” Curr. Opin. Chem. Biol. 2019, 49, A4-A5.
43. Smith, M.A.; Majer, S.H.; Vilbert, A.C.; Lancaster, K.M. “Controlling a burn: outer-sphere gating of hydroxylamine oxidation by a distal base in cytochrome P460.” Chem. Sci. 2019, 10, 3756-3764.
42. Gordon, J.B.; Vilbert, A.C.; Siegler, M.A.; Lancaster, K.M.; Moënne-Loccoz, P.; Goldberg, D.P. “A Nonheme Thiolate-Ligated Cobalt Superoxo Complex: Synthesis and Spectroscopic Characterization, Computational Studies, and Hydrogen Atom Abstraction Reactivity.” J. Am. Chem. Soc. 2019, 141, 3641–3653.
41. Roy, L.; Al-Afyouni, M.H.; DeRosha, D.E.; Mondal, B.; DiMucci, I.M.; Lancaster, K.M.; Shearer, J.; Bill, E.; Brennessel, W.W.; Neese, F.; Ye, S.; Holland, P.L. “Reduction of CO2 by a masked two-coordinate cobalt(I) complex and characterization of a proposed oxodicobalt(II) intermediate.” Chem. Sci. 2019, 10, 918–929.
40. Hulley, E.B.; Heins, S.P.; Wolczanski, P.T.; Lancaster, K.M.; Lobkovsky, E.B. “Azaallyl-derived ring formation via redox coupling in first row transition metals.” Polyhedron 2019, 158, 225–233.
39. Leguto, A.J.; Smith, M.A.; Morgada, M.N.; Zitare, U.A.; Murgida, D.H.; Lancaster, K.M.; Vila, A.J. “Dramatic Electronic Perturbations of CuA Centers via Subtle Geometric Changes.” J. Am. Chem. Soc. 2018, 141, 1373–1387.
38. Lancaster, K.M. “Revving up an artificial metalloenzyme.” Science 2018, 361, 1071–1072.
37. Siu, J.C.; Sauer, G.S.; Saha, A.; Macey, R.L.; Fu, N.; Chauviré, T.; Lancaster, K.M.; Lin, S. “Electrochemical Azidooxygenation of Alkenes Mediated by a TEMPO–N3 Charge-Transfer Complex.” J. Am. Chem. Soc. 2018, 140, 12511–12520.
36. Cook, B.J.; Di Francesco, G.N.; Ferreira, R.B.; Lukens, J.T.; Silberstein, K.E.; Keegan, B.C.; Catalano, V.J.; Lancaster, K.M.; Shearer, J.; Murray, L.J. “Chalcogen Impact on Covalency within Molecular [Cu3(μ3-E)]3+ Clusters (E = O, S, Se): A Synthetic, Spectroscopic, and Computational Study.” Inorg. Chem. 2018, 57, 11382–11392.
35. Dey, A.; Confer, A.M.; Vilbert, A.C.; Moënne‐Loccoz, P.; Lancaster, K.M.; Goldberg, D.P. “A Nonheme Sulfur‐Ligated {FeNO}6 Complex and Comparison with Redox‐Interconvertible {FeNO}7 and {FeNO}8 Analogues.” Angew. Chem. Int. Ed. 2018, 57, 13465–13469.
34. Bloom, A.J.; Lancaster, K.M. “Manganese Binding to Rubisco Could Drive a Photorespiratory Pathway That Increases the Energy Efficiency of Photosynthesis.” Nature Plants 2018, 4, 414–422.
33. Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; Kanatzidis, M.G.; King, P.; Lancaster, K.M.; Lymar, S.V.; Pfromm, P.; Schneider, W.F.; Schrock, R.R. “Beyond Fossil Fuel–Driven Nitrogen Transformations.” Science 2018, 360, 873.
32. Goodwin, C.A.P.; Réant, B.L.L.; Kragskow, J.G.C.; DiMucci, I.M.; Lancaster, K.M.; Mills, D.P.; Sproules, S. “Heteroleptic Samarium(III) Halide Complexes Probed by Fluorescence-Detected L3-Edge X-ray Absorption Spectroscopy.” Dalton Trans. 2018, 47, 10613–10625.
31. Broere, D.L.J.; Mercado, B.Q.; Lukens, J.; Vilbert, A.; Banerjee, G.; Lant, H.; Lee, S.H.; Bill, E.; Lancaster, K.M.; Sproules, S.; Holland, P.L. “Reversible Ligand‐Centered Reduction in Low‐Coordinate Iron Formazanate Complexes.” Chem. Eur. J. 2018, 24, 9417–9425.
30. Lancaster, K.M. “Sizing Up a Supercharged Ferryl.” Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 4532–4534.
29. Broere, D.L.J.; Mercado, B.Q.; Bill, E.; Lancaster, K.M.; Sproules, S.; Holland, P.L. “Alkali Cation Effects on Redox-Active Formazanate Ligands in Iron Chemistry.” Inorg. Chem. 2018, 57, 9580–9591.
28. Dong, M.; Kathiresan, V.; Fenwick, M.K.; Torelli, A.T.; Zhang, Y.; Caranto, J.D.; Dzikovski, B.; Sharma, A.; Lancaster, K.M.; Freed, J.H.; Ealick, S.E.; Hoffman, B.M.; Lin, H. “Organometallic and Radical Intermediates Reveal Mechanism of Diphthamide Biosynthesis.” Science 2018, 359, 1247–1250.
27. Lancaster, K.M.; Caranto, J.D.; Majer, S.H.; Smith, M.A. “Alternative Bioenergy: Updates to and Challenges in Nitrification Metalloenzymology.” Joule 2018, 2, 1–21.
26. Vilbert, A.C.; Caranto, J.D; Lancaster, K.M. “Influences of the Heme-lysine Crosslink in Cytochrome P460 over Redox Catalysis and Nitric Oxide Sensitivity.” Chem. Sci. 2018, 9, 368-379.
25. Smith, M.A.; Lancaster, K.M. “The Eponymous Cofactors in Cytochrome P460s from Ammonia-Oxidizing Bacteria Are Iron Porphyrinoids Whose Macrocycles Are Dibasic.” Biochemistry 2017, 57, 334–343.
24. Agnew, D.W.; DiMucci, I.M.; Arroyave, A.; Gembicky, M.; Moore, C.E.; MacMillan, S.N.; Rheingold, A.E.; Lancaster, K.M.; Figueroa, J.S. “Crystalline Coordination Networks of Zero-Valent Metal Centers: Formation of a 3-Dimensional Ni(0) Framework with m-Terphenyl Diisocyanides.” J. Am. Chem. Soc. 2017, 139, 17257–17260.
23. Wilding, M.J.T.; Iovan, D.A.; Wrobel, A.T.; Lukens, J.T.; MacMillan, S. N.; Lancaster, K.M.; Betley, T.A. “Direct Comparison of C–H Bond Amination Efficacy through Manipulation of Nitrogen-Valence Centered Redox: Imido versus Iminyl.” J. Am. Chem. Soc. 2017, 139, 14757–14766.
22. Walroth, R.C.; Miles, K.C.; Lukens, J.T.; MacMillan, S.N.; Stahl, S.S.; Lancaster, K.M. “Electronic Structural Analysis of Copper(II)–TEMPO/ABNO Complexes Provides Evidence for Copper(I)–Oxoammonium Character.” J. Am. Chem. Soc. 2017, 139, 13507–13517.
21. Obanda, A.; Martinez, K.; Schmehl, R.H.; Mague, J.T.; Rubtsov, I.V.; MacMillan, S.N.; Lancaster, K.M.; Sproules, S.; Donahue, J.P. “Expanding the Scope of Ligand Substitution from [M(S2C2Ph2)] (M = Ni2+, Pd2+, Pt2+) To Afford New Heteroleptic Dithiolene Complexes.” Inorg. Chem. 2017, 56, 10257–10267.
20. Caranto, J.D.; Lancaster, K.M. “Nitric Oxide is an Obligate Bacterial Nitrification Intermediate Produced by Hydroxylamine Oxidoreductase.” Proc. Natl. Acad. Sci. U.S.A. 2017, 31, 8217–8222.
19. MacMillan, S.N.; Lancaster, K.M. “X-ray Spectroscopic Interrogation of Transition-Metal-Mediated Homogeneous Catalysis: Primer and Case Studies.” ACS Catal. 2017, 7, 1776–1791.
18. Caranto, J.D.; Vilbert, A.C.; Lancaster, K.M. “Nitrosomonas Europaea Cytochrome P460 is a Direct Link Between Nitrification and Nitrous Oxide Emission.” Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 14704–14709.
17. Ferrando-Soria, J.; Magee, S.A.; Chiesa, A.; Carretta S.; Santini, P., Vitorica-Yrezabal, I.J.; Tuna, F.; Whitehead, G.F.S.; Sproules, S.; Lancaster, K.M.; Barra, A-L.; Timco, G.A.; McInnes, E.J.L.; Winpenny, R.E.P. “Switchable Interaction in Molecular Double Qubits.” Chem 2016, 1, 727–752.
16. Varela-Álvarez, A.; Yang, T.; Jennings, H.; Kornecki, K.P.; MacMillan, S.N.; Lancaster, K.M.; Mack, J.B.C.; Du Bois, J.; Berry, J.F.; Musaev, D.G. “Rh2(II,III) Catalysts with Chelating Carboxylate and Carboxamidate Supports: Electronic Structure and Nitrene Transfer Reactivity.” J. Am. Chem. Soc. 2016, 138, 2327–2341.
15. Walroth, R.C.; Lukens, J.T.; MacMillan, S.N.; Finkelstein, K.D.; Lancaster, K.M. “Spectroscopic Evidence for a 3d10 Ground State Electronic Configuration and Ligand Field Inversion in [Cu(CF3)4]1-.” J. Am. Chem. Soc. 2016, 138, 1922–1931.
14. Corcos, A.R.; Villanueva, O.; Walroth, R.C.; Sharma, S.K.; Bacsa, J.; Lancaster, K.M.; MacBeth, C.E.; Berry, J.F. “Oxygen Activation by Co(II) and a Redox Non-Innocent Ligand: Spectroscopic Characterization of a Radical–Co(II)–Superoxide Complex with Divergent Catalytic Reactivity.” J. Am. Chem. Soc. 2016, 138, 1796–1799.
13. Hulley, E.B.; Williams, V.A.; Hirsekorn, K.F.; Wolczanski, P.T.; Lancaster, K.M.; Lobkovsky, E.B. “Application of 93Nb NMR spectroscopy to (silox)3Nb(Xn/Lm) complexes (silox = tBu3SiO): Where does (silox)3Nb(NN)Nb(silox)3 appear?” Polyhedron 2016, 103, 105–114.
12. Zeng, T.; Lancaster, K.M.; Ananth, N.; Hoffmann, R. “Anomalous Orbital Admixture in Ammine Complexes.” J. Organomet. Chem. 2015, 792, 6–12.
11. Walroth, R.C.; Uebler, J.W.H.; Lancaster, K.M. “Probing CuI in Homogeneous Catalysis Using High-Energy-Resolution Fluorescence-Ddetected X-ray Absorption Spectroscopy.” Chem. Commun. 2015, 55, 9864–9867.
10. Yao, S.A.; Martin-Diaconescu, V.; Infante, I.; Lancaster, K.M.; Gotz, A.W.; DeBeer, S.; Berry, J.F. “Electronic Structure of Ni2E2 Complexes (E = S, Se, Te) and a Global Analysis of M2E2 Compounds: A Case for Quantized E2n- Oxidation Levels with n = 2, 3, or 4.” J. Am. Chem. Soc. 2015, 137, 4993–5011.
9. Morsing, T.J.; MacMillan, S.N.; Uebler, J.W.H.; Brock-Nannestad, T.; Bendix, J.; Lancaster, K.M. “Stabilizing Coordinated Radicals via Metal-Ligand Covalency: A Structural, Spectroscopic, and Theoretical Investigation of Group 9 Tris(dithiolene) Complexes.” Inorg. Chem. 2015, 54, 3660–3669.
8. MacMillan, S.N.; Walroth, R.C.; Perry, D.M.; Morsing, T.J.; Lancaster, K.M. “Ligand-Sensitive but Not -Diagnostic: Evaluating Cr Valence-to-Core X-ray Emission Spectroscopy as a Probe of Inner-Sphere Coordination.” Inorg. Chem. 2015, 54, 205–214.
7. Jayarathne, U.; Chandrasekharan, P.; Green, A.F.; Mague, J.T.; DeBeer, S.; Lancaster, K.M.; Sproules, S.; Donahue, J.P. “X-ray Absorption Spectroscopy Systematics at the Tungsten L-Edge.” Inorg. Chem. 2014, 53, 8230–8241.
6. Lancaster, K.M. “Copper Protein Variants: ‘Type Zero’ Sites.” In Encyclopedia of Inorganic and Bioinorganic Chemistry 2014, 1–6.
5. Kornecki, K.P.; Briones, J.F.; Boyarskihk, V.; Fullilove, F.; Autschbach, J.; Schrote, K.E.; Lancaster, K.M.; Davies, H.M.L.; Berry, J.F. “Direct Spectroscopic Characterization of a Transitory Dirhodium Donor-Acceptor Carbene Complex.” Science 2013, 342, 351–354.
4. Williams, V.A.; Hulley, E.B.; Wolczanski, P.T.; Lancaster, K.M.; Lobkovsky, E.B. “Exploring the Limits of Redox Non-innocence: Pseudo Square Planar [{κ4-Me2C(CH2N=CHpy)2}Ni]n (n = 2+, 1+, 0, -1, -2) Favor Ni(II).” Chem. Sci. 2013, 4, 3636–3648
3. Palmer, J.H.; Lancaster, K.M. “Molecular Redox: Revisiting the Electronic Structures of the Group 9 Metallocorroles.” Inorg. Chem. 2012, 51, 12473–12482.
2. Warren, J.J.; Lancaster, K.M.; Richards, J.H.; Gray, H.B. “Inner- and Outer-Sphere Metal Coordination in Blue Copper Proteins. J. Inorg. Biochem. 2012, 115, 119–126.
1. Lancaster, K.M. “Biological Outer Sphere Coordination.” Struct. Bond. 2011, 142, 119–153.
Neu, H.M.; Quesne, M.G.; Yang, T.; Prokop-Prigge, K.A.; Lancaster, K.M.; Donohoe, J.; DeBeer, S.; de Visser, S.P.; Goldberg, D.P. “Dramatic Influence of an Anionic Donor on the Oxygen-Atom-Transfer Reactivity of an Mn(V)-Oxo Complex.” Chem. Eur. J. 2014, 20, 14584–14588.
Pollock, C.J.; Lancaster, K.M.; Finkelstein, K.D.; DeBeer, S. “Angular Dependence of Valence-to-Core X-ray Emission Spectra.” Inorg. Chem. 2014, 53, 10378–10385.
Pollock, C.J.; Tan, L.L.; Zhang, W.; Lancaster, K.M.; Lee, S.; DeBeer, S. “Light Atom Influences on the Electronic Structures of Iron-Sulfur Clusters.” Inorg. Chem. 2014, 53, 2591–2597.
Yan, Y.; Keating, C.; Chandrasekharan, P.; Jayaranthne, U.; Mague, J.T.; DeBeer, S.; Lancaster, K.M.; Sproules, S.; Rubtsov, I.G.; Donahue, J.P. “Ancillary Ligand Effects upon Dithiolene Redox Noninnocence in Tungsten Bis(dithiolene) Complexes.” Inorg. Chem. 2013, 52, 6743-6751.
Lancaster, K.M.; Hu, Y.; Bergmann, U.; Ribbe, M.W.; DeBeer, S. “X-ray Spectroscopic Observation of an Interstitial Carbide in NifEN-Bound FeMoco Precursor.” J. Am. Chem. Soc. 2013, 135, 610-612.
Kropp, H.; King, A.E.; Khusniyarov, M.M.; Heinemann, F.W.; Lancaster, K.M.; DeBeer, S.; Bill, E.; Meyer, K. “Manganese Nitride Complexes in Oxidation States III, IV, and V: Synthesis and Electronic Structure.” J. Am. Chem. Soc. 2012, 134, 15538-15544.
Yao, S.A.; Lancaster, K.M.; DeBeer, S.; Berry, J.F. “Characterization and Reactivity of a Selenium-Selenium Half Bond: A New Chemical Paradigm for the Chalcogens.” Chem. Eur. J. 2012, 18, 9179-9183.
Scarborough, C.C.; Lancaster, K.M.; DeBeer, S.; Weyhermüller, T.; Sproules, S.; Wieghardt, K. “Experimental Fingerprints for Redox-Active Terpyridine in [Cr(tpy)2](PF6)n (n = 3-0), and the Remarkable Electronic Structure of [Cr(tpy)2]1-” Inorg. Chem. 2012, 51, 3718-3732.
Lancaster, K.M.; Roemelt, M.; Ettenhuber, P.; Hu, Y.; Ribbe, M.W.; Neese, F.; Bergmann, U.; DeBeer, S. “X-ray Emission Spectroscopy Evidences Interstitial Carbide in Nitrogenase Iron-Molybdenum Cofactor.” Science 2011, 334, 974-977.
Lancaster, K.M.; Finkelstein, K.D.; DeBeer, S. “Kß X-ray Emission Spectroscopy Offers Unique Chemical Bonding Insights: Revisiting the Electronic Structure of Ferrocene.” Inorg. Chem. 2011, 50, 6767-6774.
Lancaster, K.M.; Zaballa, M.E.; Sproules, S.; Sundararajan, M.; DeBeer, S.; Richards, J.H.; Vila, A.J.; Neese, F.N.; and Gray, H.B. “Outer-Sphere Contributions to the Electronic Structure of Type Zero Copper Proteins” J. Am. Chem. Soc. 2012, 134, 8241-8253.
Potapov, A.; Lancaster, K.M.; Richards, J.H.; Gray, H.B.; Goldfarb, D. “Spin Delocalization over the Type Zero Copper Site.” Inorg. Chem. 2012, 51, 4066-4075.
El Nahhas, A.; Consani, C.; Blanco-Rodríguez, A.M.; Lancaster, K.M.; Braem, O.; Cannizzo, A.; Towrie, M.; Clark, I.P.; Zális, S.; Chergui, M. ; Vlcek, A., Jr. “Ultrafast Excited-State Dynamics of Rhenium(I) Photosensitizers [Re(Cl)(CO)3(N,N) and [Re(imidazole)(CO)3(N,N)]+: Diimine Effects.” Inorg. Chem. 2011, 50, 2932-2943.
Lancaster, K.M.; Farver, O.; Wherland, S.; Crane, E.J. III; Pecht, I.; Richards, J.H.; and Gray, H.B. “Electron Transfer Reactivity of Type Zero Pseudomonas aeruginosa Azurin.” J. Am. Chem. Soc. 2011, 133, 4865-4873.
Lancaster, K.M.; Sproules, S.; Palmer, J.H.; Richards, J.H.; Gray, H.B. “Outer-Sphere Effects on Reduction Potentials of Copper Sites in Proteins: The Curious Case of High Potential Type 2 C112D/M121E Pseudomonas aeruginosa Azurin.” J. Am. Chem. Soc. 2010, 132, 14590-14595.
Lancaster, K.M.; Gerken, J.B.; Durrell, A.C.; Palmer, J.H.; Gray, H.B. “Electronic Structures, Photophysical Properties, and Electrochemistry of Ruthenium(II)(bpy)2 Pyridylimidazole Complexes.” Coord. Chem. Rev. 2010, 254, 1803-1811.
Lancaster, K.M.; DeBeer George, S.; Yokoyama, K.; Richards, J.H.; and Gray, H.B. “Type Zero Copper Proteins.” Nature Chem. 2009, 1, 711-715.
Palmer, J.H.; Mahammed, A.; Lancaster, K.M.; Gross, Z.; and Gray, H.B. “Structures and Reactivity Patterns of Group IX Metallocorroles.” Inorg. Chem. 2009, 48, 9308-9315.
Lancaster, K.M.; Yokoyama, K.; Richards, J.H.; Winkler, J.R.; Gray, H.B. “High Potential C112D/M121X (X = M, E, H, L) Pseudomonas aeruginosa Azurins.” Inorg. Chem. 2009, 48, 1278-1280.
Davis, C.; Murphy R.; Lancaster, K.M.; Devendra G.; Crane, E.J. III “A Mechanistic Comparison of the Pyrococcal NADH Oxidase and Coenzyme A Disulfide Reductase: Two Hyperthermophilic Enzymes that are Similar but Different.” in Flavins and Flavoproteins 2005 (Nishino, T., Miura, R. and Tanokura, M., Eds.) ArchTect Inc., Tokyo Japan (2005).
Hummel, C.S.; Lancaster, K.M.; and Crane, E.J. III “Determination of Coenzyme A Levels in Pyrococcus furiosus and other Archaea: Implications for a General Role of Coenzyme A in Thermophiles.” FEMS Microbiological Letters 2005, 252, 229-234.
Harris, D.R.; Ward, D.E.; Feasel, J.T.; Lancaster, K.M.; Murphy, R.D.; Mallet, T.C.; and Crane, E.J. III “Discovery and Characterization of a Coenzyme A Disulfide Reductase from Pyrococcus horikoshii: Implications for the Disulfide Metabolism of Anaerobic Hyperthermophiles.” FEBS Journal 2005, 272, 1189-1200.